
Has a low potential for abuse relative to those in schedule 4. Abuse may lead to limited physical dependence or psychological dependence relative to those in schedule 3. It has a currently accepted medical use in treatment in the United States. Has a low potential for abuse relative to those in schedule 3. Abuse may lead to moderate or low physical dependence or high psychological dependence. Has a currently accepted medical use in treatment in the United States. Has a potential for abuse less than those in schedules 1 and 2. Abuse may lead to severe psychological or physical dependence. Has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. There is a lack of accepted safety for use under medical supervision.
Has no currently accepted medical use in treatment in the United States. Is not subject to the Controlled Substances Act. The schedule may depend on the exact dosage form or strength of the medication. Studies in animals or humans have demonstrated fetal abnormalities and/or there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience, and the risks involved in use in pregnant women clearly outweigh potential benefits.
There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use in pregnant women despite potential risks.
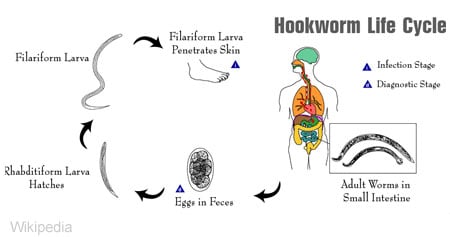
:max_bytes(150000):strip_icc()/ancylostoma-hookworm--illustration-1155265552-84ea962cc3dc403eb9bcb3894e1dd568.jpg)
This medication may not be approved by the FDA for the treatment of this condition.Īn Emergency Use Authorization (EUA) allows the FDA to authorize unapproved medical products or unapproved uses of approved medical products to be used in a declared public health emergency when there are no adequate, approved, and available alternatives.Īdequate and well-controlled studies have failed to demonstrate a risk to the fetus in the first trimester of pregnancy (and there is no evidence of risk in later trimesters).Īnimal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women.Īnimal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use in pregnant women despite potential risks. For ratings, users were asked how effective they found the medicine while considering positive/adverse effects and ease of use (1 = not effective, 10 = most effective).Īctivity is based on recent site visitor activity relative to other medications in the list.


 0 kommentar(er)
0 kommentar(er)
